
A staff member of Sinovac Biotech, a Chinese biopharmaceutical company, displays a dose of COVID-19 inactivated vaccine in Schering bottle package in Beijing, capital of China, Dec. 23, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)

A staff member of Sinovac Biotech, a Chinese biopharmaceutical company, cultivates Vero cells for production of COVID-19 inactivated vaccines in Beijing, capital of China, March 23, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)

A staff member of Sinovac Biotech, a Chinese biopharmaceutical company, cultivates Vero cells for production of COVID-19 inactivated vaccines in Beijing, capital of China, March 23, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)

Staff members of Sinovac Biotech, a Chinese biopharmaceutical company, carry out simulated novel coronavirus inoculation at a high-level biosafety zone of a stock solution workshop to be put into production of COVID-19 inactivated vaccines in Beijing, capital of China, July 15, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)
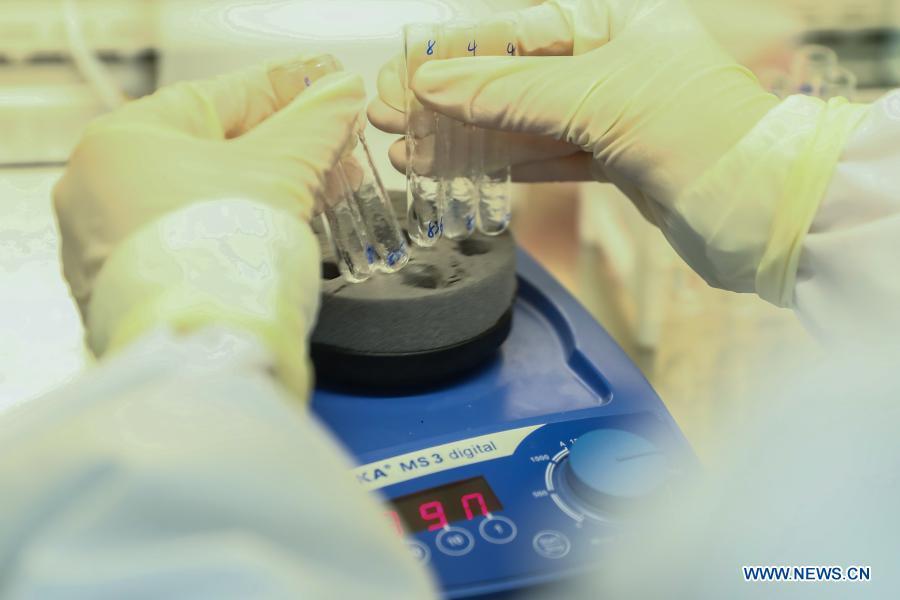
A staff member of Sinovac Biotech, a Chinese biopharmaceutical company, works in the quality inspection lab of COVID-19 inactivated vaccines in Beijing, capital of China, Dec. 23, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)

A staff member of Sinovac Biotech, a Chinese biopharmaceutical company, displays two doses of COVID-19 inactivated vaccine in prefilled syringes in Beijing, capital of China, Dec. 23, 2020. China's National Medical Products Administration on Friday granted conditional market approval to CoronaVac, an inactivated COVID-19 vaccine developed by Sinovac Biotech, the company said on Saturday. (Xinhua/Zhang Yuwei)























